Abstract
Humans migrating out of Africa encountered new conditions, including living at high altitude. Tibetans and Andean Aymaras have inhabited regions of 4,000 meters or more for ~44,000 and 14,000 years respectively (Hu et al, PLoS Genet 2017, Rademaker et al, Science 2014). There is a distinct difference in erythroid phenotypes: Aymaras are polycythemic at high altitude, while most Tibetans are not. Mutations providing an advantage in highlanders will improve fitness under hypoxic conditions,including modulation of erythropoiesis through the hypoxia-inducible factor (HIF) pathway. There are few shared, naturally-selected gene regions in Aymaras and Tibetans and these have different phenotypic associations (Bigham et al, Am J Hum Biol 2013). Aymara high-altitude selected haplotypes have not been published. Tibetan EPAS 1 (encoding HIF2a protein) haplotypes in part originated from ancient Denisovans and entered the Tibetan genome through introgression (Huerta-Sánchez et al, Nature 2014). Tibetan variant prolyl hydroxylase 2 (PHD2), a negative regulator of HIFs encoded by EGLN1 gene, encodes in cis both PHD2D4E and PHD2C127S which together have increased activity in hypoxia (Lorenzo et al, Nature Genetics 2014) and, together with a Tibetan EPAS 1 haplotype, protects from high altitude polycythemia (Tashi et al, JMM 2017). We and others previously identified other Tibetans haplotypes that are not unique but are enriched in Tibetans, including a "Tibetan" haplotype of PKLR encoding liver- and red cell-specific pyruvate kinase (PK) (Simonson et al, Science 2010, and Yi et al, Science 2010).
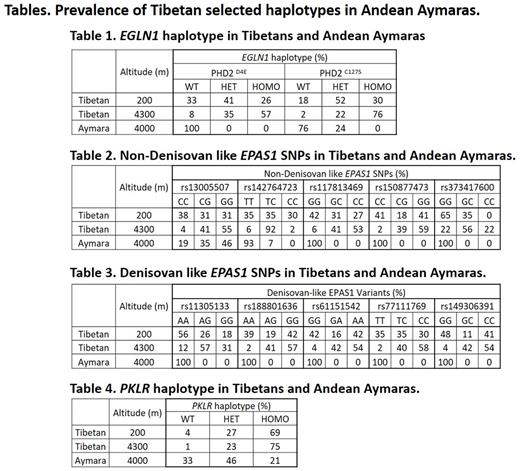
We studied Tibetan-enriched haplotypes of EGLN1, EPAS1 and PKLR in 72 Bolivian Andean Aymaras, all residing at 4,000 m, and compared them with 347 Tibetans living at altitudes of 200 m and 4,300 m (Table). We genotyped PHD2D4E and PHD2C127S variants of EGLN1 and 10 Tibetan specific single nucleotide polymorphisms (SNPs) of EPAS1, 5 Denisovan and 5 non-Denisovan, each under different linkage disequilibrium (Hu et all, PLoS Genet 2017), 7 Tibetan enriched PKLR SNPs, and searched for Aymara selected variants by whole genome sequencing.
The prevalence of the Tibetan-selected EGLN1 and EPAS1 haplotypes increased with increasing altitude of residence in Tibetans, suggesting a continuous evolutionary advantage (Tashi et al, JMM 2017). Aymaras did not have the PHD2D4E haplotype, and PHD2C127S was found at lower prevalence in heterozygote form. Aymaras shared two of five non-Denisovan EPAS1 SNPs selected in Tibetans; one, rs130005507 G allele, had a similar prevalence to Tibetans, but another, rs142764723 C allele, had a lower prevalence. We report that >90% of Tibetans and ~50% of Aymaras, but only ~10% of Europeans, have the "Tibetan " PKLR haplotype. Aymara females with homozygous PKLR haplotypes have lower hemoglobins than heterozygotes (p=0.022). Further, the PKLR transcript in reticulocytes decreases with increasing altitude and this progressive decrease is even more pronounced in the "Tibetan" haplotype. We found Aymaras' selected haplotypes encoding BRINP3, NOS2, TBX5 ; these genes are associated with cardiovascular development and function but not hypoxia sensing. They are not enriched in Tibetans.
We conclude that Aymara highlanders do not have the Tibetan PHD2D4E mutation or Denisovan-like EPAS1 variants. Furthermore, they share only two of five Tibetan non- Denisovan EPAS1 variants. The absence of these variants in Aymaras supports that Tibetan and Aymara high altitude inhabitants do not have the same ancestry, that they developed different evolutionary adaptations (Bigham et al, Am J Hum Biol 2013), and that Tibetans' EGLN1 and EPAS1 mutations are unique to that part of the world. We hypothesize that decreased PK enzyme activity would be expected to increase 2,3-diphosphoglycerate (2,3-DPG) (as is shown in people with PK enzyme deficiency) and thus be beneficial to high altitude adaptation by progressively augmenting tissue oxygen delivery with increasing altitude. Based on its association with lower hemoglobin in Aymara females, the selected "Tibetan" PKLR haplotype, present in about half of Aymaras, may contribute to their hypoxic adaptation. Additional evaluation of evolutionary selected genes including PKLR, BRINP3, NOS 2, and TBX5 and their functional consequences are in progress with Aymaras living at El Alto (4,150 m), Cochabamba (2,500 m) and Santa Cruz, (416m), Bolivia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal